by: Emily Coe and Stacy E. Croteau, MD, MMS
Published: Lifelines for Health Spring 2017
The Clinical Trial Process
Before drugs and therapeutic devices are made available to individuals to treat a disease or medical condition, the treatments are subjected to a rigorous clinical trial process in an effort to test both safety and effectiveness. Clinical research study is an umbrella term that includes a variety of different types of patient-focused studies. Interventional trials (including clinical trials) involve making an intervention to typical patient care such as the introduction of a new drug or device. Observation research studies are also important in helping us learn more about diseases and clinical care, but in these studies researchers typically collect data on participants. This can include information about medical treatments received, disease outcomes overtime, and may include surveys of patient’s report of quality of life or disease burden.
To initiate a clinical trial, a sponsor, typically a drug manufacturer and a medical researcher associated with a hospital/academic institution, needs to decide that there is evidence (data) that a drug may be of clinical use for a specific disease or group of diseases. In order to obtain permission to proceed with studying this drug in humans, the sponsor must get approval from the Food and Drug Administration (FDA) and file an Investigational New Drug (IND) application. This application includes several components such as a Study Protocol and Investigator’s Brochure (IB). The Study Protocol details the sponsor’s plan to study the new investigational (experimental) drug or therapy while monitoring its safety and effectiveness. The IB describes the pre-clinical studies that investigate the chemical properties of the drug, as well as the results of animal toxicology studies and any in-human study data to date. The FDA then reviews this application to ensure that potential participants are not at an unreasonable risk.
If granted permission to proceed, the sponsor will then identify study sites (medical centers) to carry out the clinical trial. The sponsor manages all of the primary clinical operations, ensuring proper data management and coordination across all clinical sites so the study protocol is followed correctly. The study sites identify patients who may be eligible to participate in the clinical trial, and if patients enroll on the clinical trial, the study site serves as both a treatment team as well as an intermediary between the participant and the sponsor. This protects the individual’s privacy and ensures that the highest standards of medical care are maintained during participation on the clinical trial.
The purpose of a clinical trial is to advance the current standard of medical care and to offer new and potentially more effective treatment options in a safe and objectively monitored setting. As a drug progresses in development, it moves through different phases of clinical trials as described in more detail next.
Key Terminology of Clinical Trials
The selection of study sites is a joint decision between the sponsor and the medical center. The medical center must decide if they have any patients who might be eligible for the specific study protocol, whether they have any trials already open that might compete for the same type of patients, and whether they have enough research team personnel to open the trial. The sponsor determines thenumber of participants needed to complete their study and selects study sites by the number of patients they anticipate, may participate, and the presence of research infrastructure.
Before a clinical trial can open for enrollment at a study site, the study protocol and informed consent form must be reviewed and approved by the local/site Institutional Review Board (IRB). The IRB is charged with protecting the interests of human subjects. This group carefully reviews the study protocol and other documentation from the sponsor including pre-clinical data and, if available, clinical data, which describes the known safety, toxicity and effectiveness of a product in animals and healthy human volunteers. Key components of the study protocol include the purpose of the study, the scientific rationale, the specific disease group and corresponding patient population, and the patient monitoring plan. To be eligible for participation, a patient must meet the inclusion and exclusion criteria provided by the sponsor. The inclusion criteria define the disease and age group of interest while the exclusion criteria focus on specific health states that may put patients at unreasonable risk for receiving the study drug (i.e. poor kidney, liver, or heart function). Once the study has been approved by the site’s research team and IRB, the research team can begin recruiting patients to participate.
Upon identifying eligible patients, the research team must approach the patient in order to discuss the research and obtain consent for participation in the study. An informed consent is a document that describes the details of the study, the purpose of the study, the assessments required during the duration of the study, and any known side effects of previous participants; all of which needs to be written in common, plain language. The informed consent must provide all details about what is required by a study participant including the potential risks and benefits of the study. This allows a patient to make an informed decision before agreeing to participate. A signed informed consent is required to participate in a research study.
Furthermore, if the patient is under eighteen years of age, the parent or legal guardian must sign the consent form. However, the patient must still be part of the informed consent process and sign an assent form to participate. Despite signing an informed consent document, a participant can decide to discontinue study participation at any time or can be removed at the investigator’s discretion. This may occur if the participant develops significant side effects during the study, feels the study is too much of a commitment, or is not following the study requirements (such study visits, questionnaire completion or adherence to medication regimen). Patient safety and patient choice are paramount concepts in clinical trials.
Throughout the clinical trial a participant will interact directly with the study team. This group of individuals, typically consisting of a physician, or principal investigator when leading a research team, and a study coordinator or study nurse, will facilitate and participate in study visits, ensure all required tests and surveys are completed, and help to schedule other study visits or procedures as needed.
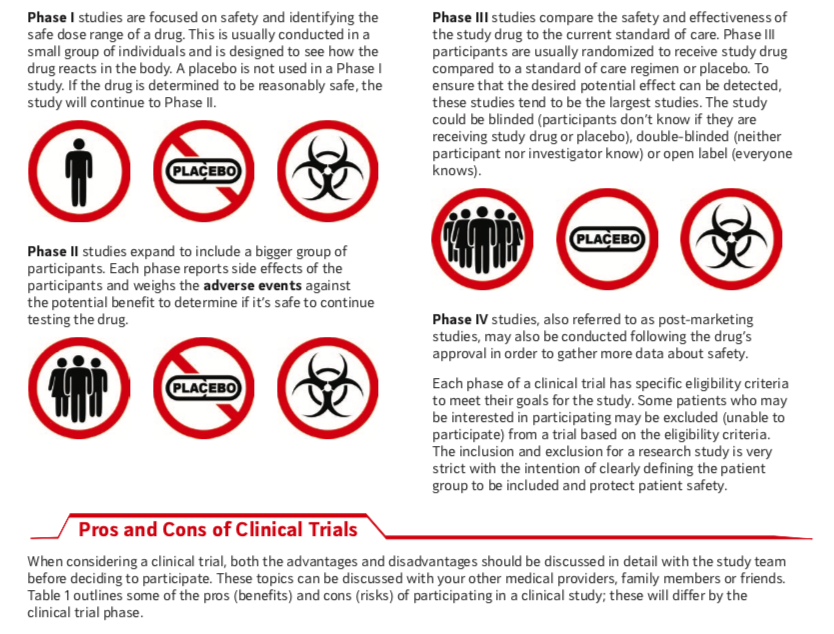
Phases of a Trial
Clinical trials are typically conducted in phases. Each phase builds upon the data provided by the prior phase and seeks to answer a different question.
Special FDA Designations
The clinical data gathered during the different phases of clinical studies are prepared as part of the submission to the FDA. This application, the New Drug Application (NDA), is submitted once the drug manufacturer or researcher believes the data is robust enough, proving safety and effectiveness of the drug, for market approval. The FDA has implemented various pathways to expedite drug development and review for serious/life threatening conditions or unfulfilled medical needs. This can be requested by the sponsor if the drug has the potential to be lifesaving or life changing, which ensures the drug is available to the public as quickly as possible.
Fast Track Designation
If the drug treats a serious/life threatening disease or is believed to fulfill an unmet need for a population, the sponsor can request consideration for a Fast Track Designation. Nonclinical or clinical data from clinical trials is needed to support this request. If granted the FDA will review the application within a few months of submission. This enables a drug that has potential to be lifesaving or life changing to be available to the market for its intended population as quickly as possible.
Breakthrough Therapy Designation
Similar to fast track, a Breakthrough Therapy Designation is granted to drugs that demonstrate significant improvement in clinical outcomes in a serious disease compared to available therapies with preliminary clinical data.
Accelerated Approval
An Accelerated Approval can be requested if the drug or therapy is shown to have a clinical benefit or advantage over current therapies. This process allows a pharmaceutical company to market the drug to the indicated population, while continuing to conduct the research study. The FDA may grant a Priority Review when filing the NDA if the clinical data demonstrates a benefit for a serious condition.
Orphan Drug Status is another incentive for drug development in rare diseases, like hemophilia. This designation provides additional support from the FDA, which includes tax incentives and grants that offset the costs and allow for market exclusivity for seven years following approval. The approval process is also expedited.
Current Clinical Trials in Hemophilia
More than 100 clinical trials are currently open and listed on clinicaltrials.gov for patients with hemophilia. These studies, as well as dozens that have been completed in the past few years, reflect a potential transformation in our approach to hemophilia treatment. Although routine (preventative) factor replacement has dramatically improved health outcomes and reduced debilitating, chronic joint damage in this population, a number of challenges remain with the use of factor concentrates to treat hemophilia A and B. Reliable intravenous access, the frequency of infusions necessary to maintain desired factor levels, and development of neutralizing alloantibodies (“inhibitors”) are among the biggest barriers. New hemophilia therapies aim to improve on our current approach of factor replacement in a number of different ways. Some products extend the circulation time of factor protein [extended half-life (EHL) products], others attempt to introduce the genetic material (DNA) necessary to allow the body to produce the missing factor protein [gene therapy, gene editing], and still others take innovative approaches to either mimic the function of the missing factor VIII protein or disrupt the protein responsible for regulating the formation of blood clots. A reduction or elimination of the need for intravenous factor concentrate or the option of a subcutaneous injection rather than an intravenous infusion are exciting prospective therapeutic options for both hemophilia patients with and without inhibitors.
Extended half-life (EHL) factor concentrates
Several EHL factor VIII and IX concentrates have completed clinical trials and have
been approved in adults and older children. The efficacy, safety, and immunogenicity (inhibitor risk) of these products continues to be investigated in our youngest hemophilia patients (previously untreated patients, PUPs). Modifications have been made to the factor VIII and factor IX proteins to extend the length of time they circulate in the blood and are available to participate in blood clot formation. The precise modification made to prolong the circulation time of the factor depends on the specific product.
Despite evidence that these products do not seem to increase the risk of inhibitor development in patients who have never had an inhibitor previously and have had more than 150 exposure days to a prior factor product, studies to investigate the risk of inhibitor development in PUPs are still ongoing, [NCT01493778, NCT02234323, NCT02615691, NCT01712438, NCT01311648, NCT02172950, NCT02137850, NCT02053792, NCT02141074]. Additionally, researchers are also exploring whether these new products can help achieve faster and overall more successful immune tolerance for patients with inhibitors [NCT02196207].
Gene therapy and gene editing
Hemophilia A and B are ideal candidates for gene therapy or gene editing because the deficiency in factors FVIII and FIX arise from a defect in a single gene. A small increase in the body’s ability to make these clotting proteins can reduce a patient’s bleeding symptoms. Most of the current gene therapy programs are investigating the safety and efficacy of gene therapy in adult hemophilia patients without inhibitors; however, potential opportunities for gene therapy/editing in patients with inhibitors using cell-based gene therapy and editing techniques are being pursued, [clinicaltrials. gov: NCT02576795, NCT02484092, NCT02618915, NCT02396342, NCT00979238, NCT02695160, NCT03003533].
Nonfactor Replacement Therapy
Therapeutic products in this category fall into two general types:
mimicking the co-factor function of factor VIII or
blocking or reducing the production of proteins responsible for decreasing clot generation (maintaining normal balance to prevent excessive clotting)
Emicizumab (ACE910) mimics the function of factor VIII and is presently in phase 3 clinical trials for both hemophilia patients with and without inhibitors, [NCT02622321, NCT02795767, NCT02847637]. While early data has demonstrated a remarkable decrease in bleed events, particularly for patients with inhibitors, recently reported blood clot adverse events, which arose in the setting of concomitant use of bypassing agents, have increased scrutiny of this agent. There are several investigational drugs that block or reduce anti-coagulation protein, namely tissue factor pathway inhibitor (TFPI) inhibitors (concizumab and BAY 1093884) and blocking production of antithrombin (AT) (fitusiran). TFPI plays an important role in regulating the initiation of thrombin generation by inhibition of tissue factor-factor VIIa (TF-FVIIa) and prothrombinase. For patients with hemophilia, amplification of coagulation signaling and the thrombin burst is inadequate. Products designed to block the function of TFPI are in ongoing phase I clinical trials, [NCT02571569, NCT02490787]. Both IV and SQ formulations are being studied. Antithrombin plays a direct role in inhibiting thrombin production and decreases clotting activity of other coagulation proteins. A phase I dose escalation trial investigating weekly and monthly infusion schedules of fitusiran is underway, NCT02035605. Early results show a decrease in AT levels and a corresponding decrease in annualized bleed rates and no safety concerns in the small number of patients studied so far.
While it is exciting to have so many new therapeutic products under investigation, as we discussed above, large clinical trials to explore the effectiveness and safety of these products are imperative. Early phase studies can have encouraging results, but then expansion to a larger pool of patients can demonstrate that an investigational product is not as effective as initially thought or that there are important safety considerations that were not previously identified. Until a drug is reviewed and approved by the FDA, it is important to remember that the products described above are investigational in nature and may or may not become commercially available for individuals with hemophilia. In many cases, ongoing surveillance for safety is important even after a product is approved by the FDA for use. To learn more about specific clinical trials, study sites that are actively enrolling patients, and the general inclusion and exclusion criteria, please see clinicaltrials.gov.
Editor’s note: On May 15, 2017, in Hemophilia News Today, Dimension Therapeutics announced they would end development of DTX101 as Gene Therapy for Hemophilia B.
Glossary of Helpful Clinical Trial Terms
Adverse Event: Any negative change in the health of a clinical trial participant’s health that occurs during or for a
specified period after the trial.
Antibody: A protein secreted into the blood stream to neutralize pathogens, including bacteria, viruses, or
foreign proteins. Antibodies may be designed to interact with specific proteins for therapeutic purposes.
Clinical Trial: A process of testing the safety and efficacy of a new drug in people. Divided into four
different “phases” (see below).
Cohort: A group of clinical trial research participant who share a characteristic of interest.
Double-Blind: Both the research participants and investigators do not know the natire of the intervention being administered to specific patients. A third party reveals which group received which intervention after the outcome of the trial has been assessed. Used to prevent bias when the drug being tested is compared to placebo or another approved drug.
Exclusion Criteria: Attributes that prevent an individual from participating in a clinical trial. Determined before the trial begins and cannot be altered. These are designed to keep research participants safe.
Gene Therapy or Editing: Inserting a functional copy of a gene into a patient to treat a disease.
Half-life: The amount of time it takes for the concentration of a drug to decrease by half.
Inclusion Criteria: Required attributes for individuals to participate in a clinical trial. Determined before the trial begins and cannot be altered. These are designed to define the type of patients being studied and to keep research participants safe.
Investigational New Drug (IND): An application to the FDA for permission to begin testing a drug in humans. Contains results of toxicity studies and protocols for manufacturing the drug and carrying out clinical trials. Required before a drug can enter phase clinical trials.
Intravenously: Administration of a drug directly into a vein. Investigators: A researcher who is involved in conduction a
clinical trial.
Off-Label Usage: Use of an FDA approved drug for an indication, or in a group, not approved on the prescription label.
Open-Label: Both research participants and investigators know the nature of the intervention being administrated.
Peak Level: Maximum concretion of a drug in the bloodstream of a patient after administration of one dose.
Phase I: A clinical trial in which a drug is tested on research participants to determine the maximum tolerated dose of a drug and to evaluate safety.
Phase II: A clinical trial in which a drug is tested on a small group of patients to establish whether a drug has any efficacy and further test the safety of the drug. This phase can only begin after a phase I trial has been completed successfully.
Phase III: The phase of testing for a drug can be approved. In phase III trial a drug tested on a large group of patients to further establish the efficacy of a drug in treating a disease. The data from this trial is reviewed by FDA in hopes of approval. This phase can only begin after a phase I trial has been completed successfully.
Phase IV: Continued evaluation of a drug for safety and efficacy after it has been approved for marketing. It is also known as post-marketing surveillance. Does NOT evaluate the drug for new indications.
Primary endpoint: The outcome that is measured at the end of the trial to determine if the drug was successful in treating the disease. The primary endpoint is determined before the trial begins.
RNA Interference: The process in which the production of a specific protein is decreased by an RNA molecule. RNA is a chemical “cousin” of DNA.
Single Blind: The research participants do not know the nature of the interventions being administered. Used to prevent bias when the drug being tested is compared to placebo or another drug.
Study Arm: A group of research participants that receives a specific type of intervention. Includes the drug being investigated, a placebo control, or a previously approved treatment for the same condition.
Subcutaneous: Administration of a drug under the skin.
Top Line Results: The results of a statistical analysis of clinical trial data that indicate if endpoints have been met. Examples may include showing an experimental drug is statistically superior to another already approved drug, or that an experimental drug shows no statistical difference with a placebo.
Trial Sponsor: The person or organization who initiates a clinical trial and holds the investigational new drug application. May be a governmental organization, a corporation, or an individual investigator. Trough Level: minimum concentration of a drug in a patient’s bloodstream.
This glossary was created for New England Hemophilia Association for the first of its kind, consumer medical update offered on March 25th 2017. It was created by the Consumer Medical Update Committee to help understand key terms used for clinical trials.