MOTIVATE Study: The New Era of Immune Tolerance Induction
by Dr. Robert Sidonio
Published: LifeLines for Health, vol. 18 (Fall/Winter 2021)
Hemophilia A is caused by defective or deficient coagulation factor VIII. Severity is based on the baseline FVIII level <1% (severe), 1-5% (moderate) and >5-50% (mild). Patients with severe hemophilia A are at risk for recurrent bleeding episodes including but limited to joint bleeding. Despite all the technologic improvements in manufacturing and modifications of FVIII, inhibitor development continues to be the biggest challenge in the care of hemophilia A.
In 1977 (I was a year old at the time), the first described patient with hemophilia A and an inhibitor was tolerized utilizing a protocol that Dr. Brackmann developed in Bonn Germany. The concept was simple but nevertheless elegant. The plan was to give repeated high dose exposure to FVIII to exhaust the immune system and allowing the body to accept FVIII without developing further antibodies against it.
Nearly 40 years later it remains the only effective strategy for inhibitor eradication. I recall meeting Dr. Brackmann and boldy (I know it is hard to believe) telling him that ITI in its current form is dead. After a nice discussion, we concluded - we must evolve how we eradicate inhibitors.
Since the advent of this ITI regimen, there have been only minor adjustments in the approach to ITI and still today there are many unresolved issues regarding ITI. First, is there a reason to consider ITI now with emicizumab prophylaxis available in most resource rich countries which is effective at mitigating bleeding regardless of inhibitor status? Second is, what is the role of von Willebrand factor (VWF) containing concentrates in ITI? Third, is how do we measure ITI futility and how do we define tolerance in the post-emicizumab era? In 2021 is there a role for ITI in the patient with hemophilia A with an inhibitor? Because emicizumab is the only available non-factor therapy available for clinical use we will focus on how it has changed the way we think about ITI and potentially obviates the need for inhibitor eradication.
As aforementioned it has become abundantly clear that the way we perform ITI must change in the upcoming decades. The pre-emicizumab ITI standard of care often required the use of a central line, daily to every other day high volume FVIII infusions, bypassing agent prophylaxis or BPA (either rFVIIa or aPCC) prophylaxis which puts a tremendous amount of burden on the family and patient. In the past 40 years, there have been multiple variations of ITI with three strategies remaining as the most widely utilized. They can be categorized by dose intensity or more traditionally they can be categorized by the city in which it was developed (see figure 1).
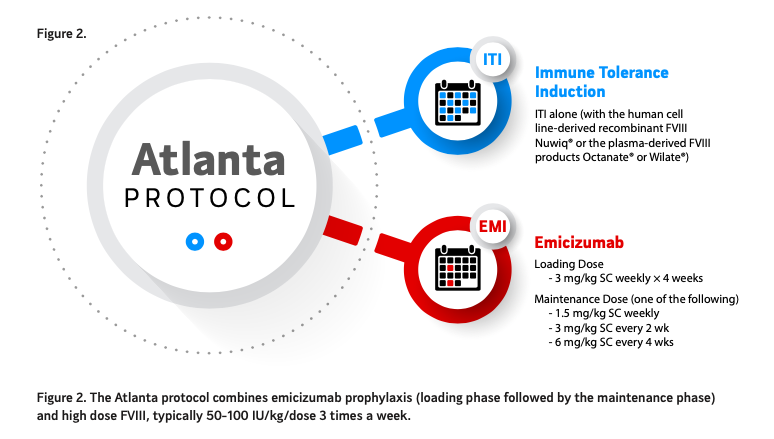
Back in 2019 our research group developed a novel way to eradicate inhibitors (see figure 2). The concept was simple; Continue emicizumab prophylaxis and modify FVIII exposure to tolerize or eradicate the inhibitor. We felt that since FVIII and emicizumab were not synergistic in a bleed control manner and would not lead to a very high level of thrombin generation we could combine the regimens safely. With the Atlanta protocol, emicizumab prophylaxis is given every week, biweekly or monthly as per label and FVIII given at 50- 100 IU/kg/dose three times a week. The product choice (class and VWF containing amount) is up to the provider and patient with no evidence of a benefit with one product type over the other. The first implementation of this protocol was conducted in a single center (our center) in seven hemophilia A patients with inhibitors, all demonstrating significant reduction of their inhibitor titer without any thrombotic or thrombotic microangiopathy (TMA) events. This new approach is called the Atlanta protocol and is under investigation with 2 clinical trials, The Emi PUPs (previously untreated patients) and Nuwiq ITI study (NCT04030052) and the MOTIVATE study. Part B of the former study is evaluating Nuwiq 100 IU/kg/ dose three times a week for 12 months while on emicizumab prophylaxis and is currently open only in the United States.
Figure 3A. The MOTIVATE study is a non-interventional study in the United States, Canada, Asia and South America and a low interventional pragmatic study focused on evaluating the safety and efficacy of the Atlanta protocol compared to standard ITI and emicizumab prophylaxis. The study is sponsored by Octapharma and is registered on www.clinicaltrials. gov.
Figure 3B. The MOTIVATE study is led by Drs. Carmen Escuriola-Ettingshausen from Germany and Robert Sidonio, Jr. from the United States at Emory University and Children’s Healthcare of Atlanta. The study design allows observational evaluation over a max of 5 years.
Patients from all groups are permitted to receive bypassing agents (aPCC or rFVIIa) if required for treatment of bleeding episodes or during surgery. Patients are followed for up to five years and may switch to another treatment group if their treatment is changed by their physician. MOTIVATE aims to enroll 120 patients of any age and with haemophilia of any severity who have developed inhibitors to any FVIII product. MOTIVATE is supported by funding from Octapharma AG (Lachen, Switzerland).
The obvious question is why should one consider ITI? Firstly, the hemostatic response to bypassing agents is less consistent and predictable and the management of surgery is more complicated particularly in mitigating the risk of thrombosis. Secondly, I personally do not think it is a good idea to rely on a single therapy for prolonged period of time. When you make the decision to not consider tolerizing, you have limited your options to one effective therapy, emicizumab. Of course, again this option is highly efficacious but it may be 2 years before another non- factor therapy option is FDA approved. It is reasonable to not consider the Atlanta protocol if you have failed ITI multiple times prior to emicizumab and have a very high titer inhibitor (>500 BU/ml). The Atlanta protocol may not be a reasonable option. However, if you have a relatively manageable inhibitor titer (<100 BU/mL) it is worthwhile o consider one attempt with the Atlanta protocol. Thirdly, future novel therapies such as AAV gene therapy or ex vivo gene therapy will not likely be options available to those with existing inhibitors thus limiting your option to future therapies. It is certainly a personal decision to consider ITI and specifically, the Atlanta protocol, but one you should consider with each visit to your local Hemophilia treatment center.
One of the challenges that remains if you have successfully undergone ITI using the Atlanta protocol is what to do once you have achieved tolerance as measured by a negative inhibitor titer, normalization of recovery and half-life. This is evolving and we have proposed a strategy of a slow wean off FVIII with careful evaluation of kinetic studies (half-life and recovery) with every wean (see figure 4). Typically, we start with three times a week weaning next to twice a week, once a week, once every other week and then off typically over 12-15 months. At this point you as the patient now have the choice to choose FVIII prophylaxis or emicizumab prophylaxis.
Whether the Atlanta protocol strategy will be fully embraced is yet to be determined but there is a strong sense of staying the course regarding attempting to tolerize every hemophilia A patient with an inhibitor. The benefit of return to use of a FVIII for bleed control and use in surgery is one major benefit, offering a choice in therapy (FVIII versus emicizumab prophylaxis) and having more options for evolving therapies such as gene therapy which soon will be approved. If it is feasible since hemophilia A with inhibitors is a relatively rare condition I hope you consider participation in a clinical trial or observational study so we can learn whether our strategy should be considered the standard of care in the near future. Either way we will continue to advocate for the hemophilia patient community and allow freedom in treatment choice.
Figure 4. Proposed algorithm following meeting successful ITI tolerance criteria. Once a patient has achieved tolerance a wean off FVIII should be done slowly likely over 12-15 months to ensure no return of the inhibitor. The goal is to stop FVIII post ITI prophylaxis and at this point consideration of FVIII prophylaxis alone or emicizumab prophylaxis can be made.