A Road of Uncertainties: Twists & Turns of Bleeding Disorder Treatments
by: Janet Brewer, M.Ed
Published: Lifelines for Health Fall/Winter 2020
The year 2020 has certainly created a wide array of confusion and unpredictable outcomes from work and school, to our home and social lives. And it doesn't stop there. Bleeding disorder treatments (old and new) have taken a few unexpected paths as well. So, buckle-up for the bumpy ride as we present some of the biggest surprises.
Novo Nordisk Concizumab Update
In the spring edition of LifeLines for Health https://ches.education/newsletter, we reported that Novo Nordisk pushed pause on 3 trial programs-Explorer 5 in phase 2 and Explorer 7 and 8 both in phase 3 due to the development of non-life threatening blood clots that developed in three (3) patients. The trials are testing the safety and efficacy of Concizumab, a subcutaneous prophylactic treatment delivered in a pen device to reduce the number of bleeds in both hemophilia A and B patients regardless of inhibitor status.
On August 13, 2020, Novo Nordisk opened up the Explorer trials five months after they were paused in March 2020. The company said in a statement. “Novo Nordisk has together with relevant authorities identified a new path forward for concizumab. New safety measures and guidelines, based on analysis of all available data, have been agreed with the FDA and the clinical hold has been lifted.”
Source: https://ml-eu.globenewswire.com/Resource/Download/32302d78-439f-4a97-a655-3f7b96e2cc76
Gene Therapy Update
A surprise ruling by the FDA on August 18, 2020 regarding BioMarin’s Biologics License Application (BLA) for hemophilia A gene therapy valoctocogene roxaparvovec (valrox), determined that the company’s application is not ready for approval.
The FDA issued a new recommendation for two years of data that provides substantial evidence of durability using Annualized Bleeding Rate (ABR) as the primary endpoint. This new recommendation had not been required previously. In November 2019, the Phase 3 study referred to as 270-201, was fully enrolled. BioMarin will be required to submit a two-year follow up in November 2021 for all participants regarding safety and efficacy.1 In March 2020, ASH Clinical News reported the pricing for valrox would be $2 million to $3 million. Annualized bleeding rates dropped from an average of 16.5 bleeding episodes per year to an average of zero, sustained over 3 years for some trial participants. Some patients however, reporting drops in FVIII levels that raises the question regarding long-term durability.2
BioMarin was poised to be the first company to release a gene therapy option for treatment for Hemophilia A. The FDA had granted valoctocogene roxaparvovec Priority Review status and Breakthrough Therapy and Orphan Drug designations on October 26, 2017.
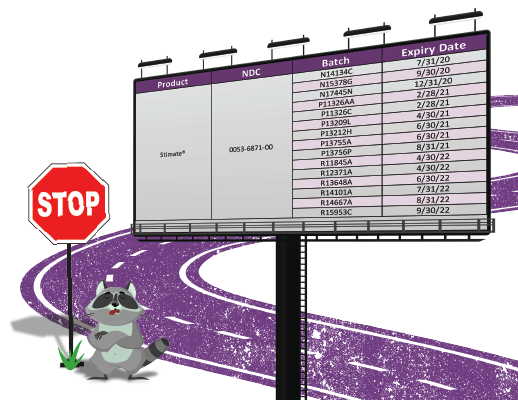
Stimate Recall
Ferring Pharmaceuticals announced a series of recall notices beginning July 21, 2020 and expanded on August 13, 2020 to include all lots of DDAVP Nasal Spray (10 mcg/0.1mL), Desmopressin Acetate Nasal Spray (10 mcg/0.1mL), and STIMATE Nasal Spray (1.5 mg/mL). Ferring manufactures Stimate and it is distributed and sold in the US by CSL Behring. The recall was necessitated due to superpotency or amounts of desmopressin higher than specified. These out of specification results were obtained during routine testing.
The Medical and Scientific Advisory Council (MASAC) of NHF issued Medical Advisory #427: Stimate Recall Update https://www.hemophilia.org/Newsroom/Medical-Advisories/Medical-Advisory-427-Stimate-Recall-Update. If you as the patient or a healthcare provider have any questions you are encouraged to call CSL’s Medical Information line at 1-800-504-5434 or email to MedinfoNA@cslbehring.com.
Patient Notification System
The Patient Notification System was established in 1998 by the Plasma Protein Therapeutics Association (PPTA) and developed by the manufacturers of plasma-derived, and recombinant analog therapies with direct input from consumers. The system is administered by Stericycle, an independent organization specializing in pharmaceutical medications. The manufacturer notifies Stericycle of the product withdrawal or recall and Stericycle notifies the patient. Your personal information is kept confidential by Stericycle. This service is free, but you MUST sign up to receive notifications at https://www.patientnotificationsystem.org/index.asp. Or 1-888-873-2838 (1-888-Update-U).
You will be asked to choose your delivery method: email, text, or phone. Email or text is highly recommended to prevent delays. Don’t forget to keep your information up to date! It is always important to keep detailed and accurate logs that include lot #s, product name and manufacturer.